HEAL Director Génon K. Jensen writes for EUobserver on the importance to reflect the heavy toll climate change, biodiversity loss and pollution are taking on health in the EU Strategic Agenda 2024-2029 .
By Natacha Cingotti (HEAL’s Health and Chemicals Lead) and Angeliki Lyssimachou (HEAL’s Senior Science Policy Officer)
Under the European Green Deal and related Farm-to-Fork strategy, the European Commission has committed to reduce the overall use and risk of all chemical pesticides by 50%, as well as the use and risk of high-risk pesticides by 50%, by 2030. At the time of writing, it remains however difficult to see how those lofty promises will become reality. By way of example, this article focuses on the controversial implementation of the ban of pesticides that are harmful to our health via our hormonal system (known as endocrine disrupting pesticides), which we believe is illustrative of the serious loopholes requiring urgent fixing in Europe’s safety assessment and regulation of harmful pesticides.
When looking at the European regulation on pesticides, the wording is clear: pesticides identified as endocrine disruptors are considered to be of equal concern as pesticides with carcinogenic properties, which is why they should be banned or severely restricted in use. These ‘cut-off criteria’ (as specified in Regulation 1107/2009, annex II, paragraph 3.6.5) state that the identification of an endocrine disrupting pesticide triggers an automatic ban, without the need for further risk assessment. This apparently progressive wording however hides major implementation loopholes, which leave human health and the environment not adequately protected from the harm caused by endocrine disrupting pesticides.
By using the example of the ongoing assessments of five substances that have long been suspected to disrupt our thyroid function, this article illustrates the extremely slow pace of and the related weakness in the implementation of Europe’s promises to protect people, animals and wildlife from effects associated to exposure to endocrine disruptors. More than one decade after the regulation entered into force, one would expect that these commitments are at least partially implemented. This is unfortunately far from the case: to date, to our knowledge, only one pesticide active substance – the fungicide Mancozeb – has been publicly identified as an endocrine disruptor for humans.
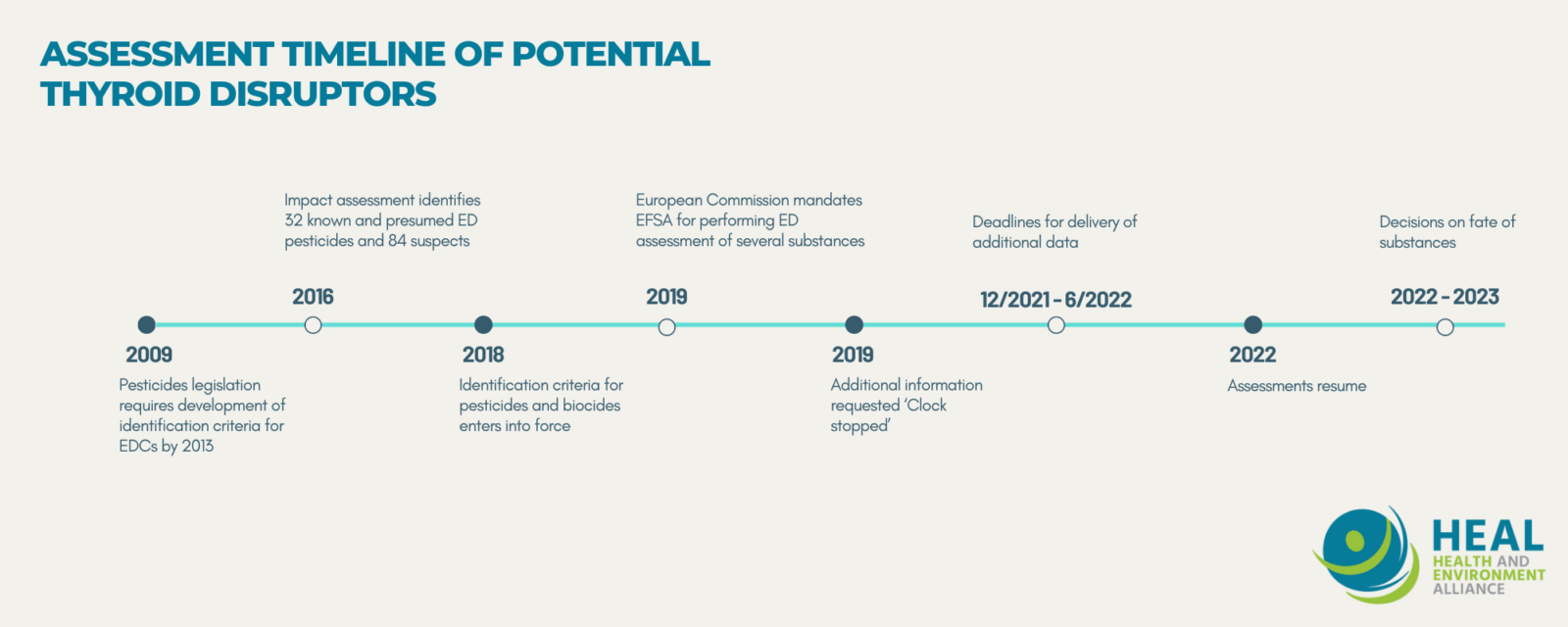
2009 – 2018: Understanding the challenge of regulating endocrine disruptors in the context of pesticide use: you can only ban what you can identify
The first loophole in the implementation of the EU ban on endocrine disrupting pesticides stems from the significant delays in the development of the scientific criteria necessary to identify an endocrine disruptor. The 2009 regulation gave the European Commission a deadline of December 2013 to put forward its proposal for such criteria (Regulation 1107/2009, annex II, paragraph 3.6.5).
The Commission’s department for Environment was ready to deliver the criteria on time, following collaboration with experts in endocrinology and human toxicology. But the process was hijacked by industry, who put heavy pressure on the Commission throughout. An intervention by the then EU Chief Scientific Advisor, triggered by a letter from scientists that were later found to have clear conflicts of interests, successfully put a halt on the development of the criteria.
On the orders of the Commission’s Secretary General, the task was handed over to the department in charge of Health and Food Safety, which was requested to carry an unforeseen impact assessment before proceeding. Much to the credit of big business-led disruption and much at the expense of public health and the environment, the delays kept accumulating, and it even took a court case initiated by Sweden before a draft proposal on the criteria finally came out of the institutions in 2016.
This time, the level of evidence required for the identification of endocrine disrupting pesticides was so high and the real-life protection potential so low, that it right away crystalized opposition from scientific and environmental health communities alike. One of the points of criticism was the lack of categories reflecting the different levels of scientific evidence available to identify endocrine disruptors, including for suspected ones. For months, the wording of the criteria was at the heart of intense debates between Member States and the Commission. The first version that was greenlighted by a majority of Member States was however vetoed by the European Parliament, as it was unacceptable from both legal and protection standpoints, sending the proposal back to the drawing board. It is only in 2017 that the criteria were adopted by all the institutions involved and they entered into force in November 2018.
All in all, the process to establish scientific criteria to identify endocrine disruptors under the pesticides legislation took no less than 9 years. So long for the urgency to act in order to prevent people’s and wildlife’s exposure to endocrine disrupting pesticides.
2018 – 2021: Implementing EDC identification criteria without appropriate data: an empty shell
When the criteria finally entered into force, HEAL, alongside our members and partners such as the EDC-Free Europe coalition were still concerned about the high and unrealistic level of evidence required to identify ‘known’ and ‘presumed’ endocrine disruptors without a category for ‘suspected’ ones. We were at least hoping that the evaluation of pesticide substances long under suspicion of having endocrine disrupting properties would finally accelerate.
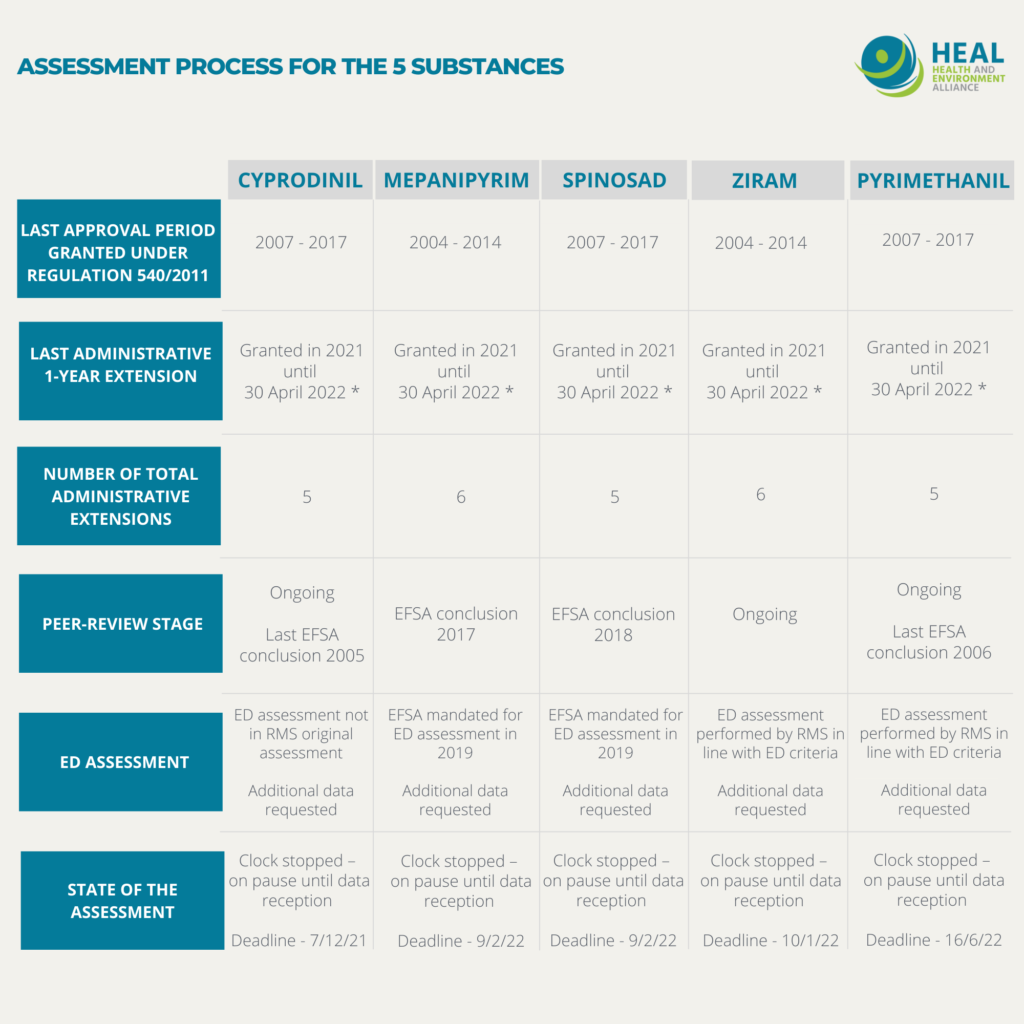
French doctors mobilized against pesticides (Alerte des Médecins sur les Pesticides – AMLP) and Générations Futures – two of HEAL’s members – were particularly interested in the outcome of the regulatory assessment of substances long flagged up for potential disruption of the thyroid axis in a report from the European Food Safety Authority (EFSA) dating back 2013. Some of those substances happen to regularly find their way into French food, according to official monitoring. Through a cross-checking exercise of EFSA and French monitoring data, we jointly extracted a number of pesticide active substances of concern – five of which we will highlight in this article: Cyprodinil, Mepanipyrim, Spinosad, Ziram, Pyrimethanil.
These five substances have all been on the European market for at least a decade and their current approval expiry dates have been extended on administrative grounds several times, prolonging their use and as a result, people’s exposure to them for multiple years. Some expiry dates have been prolonged to such an extent that the use of some pesticides has gone beyond the maximum 15-year authorization period foreseen by the regulation, irrespectively of their toxicity. For instance, Ziram is a fungicide substance that had already been flagged up as an endocrine disruptor with high potency under the 2016 impact assessment carried out by the European Commission in the preparatory phase of the EDC criteria, based on existing data. Nevertheless, its authorisation period was prolongued six times pushing forward its expiration date from 2014 to 2022, resulting in a total approval period of 18 years (see table).
When looking into endocrine disruption, disruption of the thyroid axis is particularly relevant because thyroid hormones regulate our metabolism, organ development, and their functioning (including the brain development of children). In other words, our thyroid system is an incredibly complex and sensitive system, on which any unintended effect should be considered adverse.
Because the exact stage of the assessments of pesticides substances is unclear and difficult to scrutinize for third parties, we wrote to the European Food Safety Authority (EFSA) to ask about the advancement of the renewal processes, in particular when it comes to the assessment of the endocrine disrupting properties in the light of the 2018 identification criteria and of existing concerns for thyroid disruption for all those substances (full correspondence available at the end of this article).
The responses received illustrates the safety loopholes of the current system, allowing substances to remain on the market because of a lack of data when concerns of endocrine disruption exist (see summary table below):
- For all 5 substances, due to data gaps in the industry applications, the assessment process has been put under the ‘clock stop’ procedure until the missing data is provided. This means that the timeframe foreseen in the EU pesticide regulation for carrying the assessment is suspended as long as the required endocrine disrupting-related data has not been submitted. In the meantime, the substances can remain on the market and companies can keep making profits out of their sales, while people are still being exposed.
- For 3 out of the 5 substances (Cyprodinil, Ziram, Pyrimethanil), the data has been requested directly by the rapporteur Member States in order to complete the peer-review process. Industry applicants have until end of 2021 to June 2022 to provide such data.
- For 2 out of the 5 substances (Mepanipyrim and Spinosad), EFSA was mandated to perform the assessment of endocrine disrupting properties by the EU Commission in 2019. Applicants are supposed to send the missing data to EFSA by February 2022. It is only upon receipt of this data that the assessment will resume.
- For all five substances, industry applicants have been granted the maximum possible delay (30 months) to provide additional data, despite existing concerns for thyroid effects and without publicly available reasoning for justifications. Regulation 2018/659 stipulates that such delay granting ‘shall be justified in relation to the type of information which has to be submitted’.
 * https://ec.europa.eu/transparency/comitology-register/screen/documents/071811/1/consult?lang=en
* https://ec.europa.eu/transparency/comitology-register/screen/documents/071811/1/consult?lang=en
2021 onwards: still waiting for a protective implementation of the criteria for endocrine disrupting pesticides and for full transparency on industry-submitted data
As is clear from the above, while further data is awaited in order to conclude on potential endocrine disrupting properties, all five substances are allowed to remain on the European market.
Based on the prospective timing for receipt of such data, it appears that no conclusion will be issued for any of those substances before at least spring 2022. What’s more, it is likely that their current approval expiry dates will be extended even further. This raises questions about the authorities’ sense of urgency in addressing concerns about endocrine disruption and getting the provisions of the pesticides regulation duly implemented.
Whereas it is understandable that the entry into force of the criteria to endocrine disrupting pesticides required a transition, it is striking to see that it had not been anticipated from both regulatory and industry sides. Surely these criteria only came into force in 2018, but information requirements for the testing of endocrine disrupting properties already existed before that date. This means that industry applicants could already commission such tests upon suspicion of endocrine disruption. Furthermore, we are well aware of current limitations when it comes to ED testing – for example due to limited laboratories capacities and availabilities to perform specific testing. However this is an issue that should and could have been factored in ahead of the entry into force of the criteria to avoid it being misused at the expense of health and environment protection.
All in all, three years after the introduction of the criteria to identify endocrine disrupting pesticides (and 8 years past the original deadline for their introduction), authorities are still waiting for the data necessary to their implementation. In other words, the weaknesses in the regulatory system only benefit companies who sell the substances under suspicion of health and environment harm, while exposure continues.
Highlighting the example of these five substances also raises questions about the myriad of other substances that civil society groups are not able to monitor, and for which data is also most likely lacking. How many substances are falling under the same situation? How long will it take to gather the data necessary for an assessment of all endocrine disruption suspects in line with the high burden of proof set by the criteria? How long will it take before decisions on their fate are taken and for all the substances that are of concern in terms of endocrine disruption to be kicked out of the market? The health and environmental implications are potentially significant but we have no answers to those questions.
Meanwhile, the entry into force of the Transparency Regulation in March 2021 raised high expectations from civil society groups and independent scientists in terms of their future ability to scrutinize the industry-submitted data to authorities in the course of pesticide approvals and renewals. Here again, the revolution will take longer than expected to happen. While the regulation does not apply to ‘applications under Union law as well as requests for scientific outputs submitted to the Authority before 27 March 2021’ (article 11(1) of Regulation 2019/1381), we still believe that any data that is needed to carry out the assessment of hazard properties of pesticides (such as for endocrine disruption) and is yet to be submitted after the entry into force of the regulation, should be made public. We questioned authorities about their readiness to make the data related to the ED assessment of the five substances discussed here publicly available. Unfortunately, based on the responses we have received so far, it seems this is not yet about to happen.
In HEAL’s view, the slow handling of the assessment of these 5 potential thyroid disruptors is illustrative of the inherent flaws of the criteria to identify endocrine disrupting pesticides and the current regulatory approach to identifying EDCs in general. The environmental health community has continuously warned authorities about the unrealistically high burden of proof to their implementation and pleaded for the establishment of categories to reflect the differing levels of scientific evidence to adequately identify these substances (including a category for suspected EDCs). The current delays in producing the adequate data to assess those substances bluntly demonstrate such impracticability.
Besides, the remaining lack of transparency around those assessments is unacceptable. Because those substances’ assessments have been dragging on for years and formally started before the entry into force of Europe’s Transparency Regulation (notwithstanding the absence of key data to undertake such assessments), third party observers will not be able to access the scientific studies used and to scrutinize their scientific validity real-time. We are here only presenting a limited number of cases that we have monitored, but there must be many more. We have no comprehensive information about the total number of substances, for which assessments processes started before the entry into force of the transparency provisions and which have also been put on hold due to a lack of data.
Hopefully, the current discussions kickstarting the development of identification criteria for endocrine disruptors through the European hazard classification regulation (the classification, labelling and packaging regulation) that has been announced under the Chemicals Strategy for Sustainability in October 2020 will allow for a harmonized approach to the hazard identification of endocrine disruptors across regulations in the future. However, it will take several years before such proposal is developed and implemented. In the meantime, when it comes to pesticides, substances are and will continue to be assessed under the 2018 identification criteria.
In our view, for European authorities, there are two ways to go from here in order to fulfil their own commitments under the EU Green Deal when it comes to endocrine disrupting pesticides: either consider that the absence of data does not prove safety and apply the precautionary principle in such cases, or be ready to lower the bar of evidence for the identification of endocrine disruptors. Finally, while Europe boasts about its transparency provisions, full scrutiny about the various steps of any pesticide assessment should be possible, especially when it comes to complex and long processes involving endocrine disruption, and for which key safety data is still awaited.