In December 2023, the Council of the European Union, the European Commission and the European Parliament reached an agreement on the proposed reform of the EU legislation on the classification, labelling and packaging of chemicals (CLP).
Introduced in 2008, the CLP Regulation sets out how the EU classifies and communicates about the hazardous properties of chemical substances and mixtures. The CLP has created a harmonised process for the identification and labelling of the hazardous properties of substances, which applies across all sectors and uses.
One of the main aims of the CLP is to determine whether a substance or mixture meets the classification criteria to be considered as hazardous for human or environmental health. It is therefore an essential aspect of hazard communication throughout the manufacturing, use, distribution and trade of chemicals. Under sectoral chemicals legislation, the existence of a CLP classification can trigger specific actions, including bans and restrictions. Proper hazard identification under CLP is key to guaranteeing high levels of health protection, efficiency and coherence across all EU chemicals legislations.
CLP Reform
The 2008 CLP relied heavily on companies to provide information, often at the disfavour of independent peer-reviewed data. Moreover, because toxicity studies provided by companies in the hazard classification procedure are not publicly available, it is difficult for independent scientific observers and civil society to meaningfully scrutinise the process.
The European Chemicals Strategy for Sustainability (CSS) (2020) acknowledges some of the limitations of the 2008 CLP Regulation and committed to introducing new hazard classes, amending the regulation to give the European Commission the mandate to initiate harmonised classification and for assessing existing toxicity criteria.
On 19 December 2022, the European Commission published its proposal to amend the 2008 CLP Regulation in line with the CSS. The proposal is a crucial step towards more protective and effective EU chemicals legislation. The European Parliament’s Committee on the Environment, Public Health and Food Safety (ENVI) voted in support of the amended draft proposal in September 2023. ENVI’s version was then voted on by the European Parliament in October 2023, with the majority of Members of the European Parliament also supporting the reform. In December 2023, the Council of the European Union, the European Commission and the European Parliament finalised their inter-institutional negotiations on the proposed reform.
HEAL campaigned actively to ensure that a health-protective CLP reform comes into force, and welcomes the outcome of the negotiations:
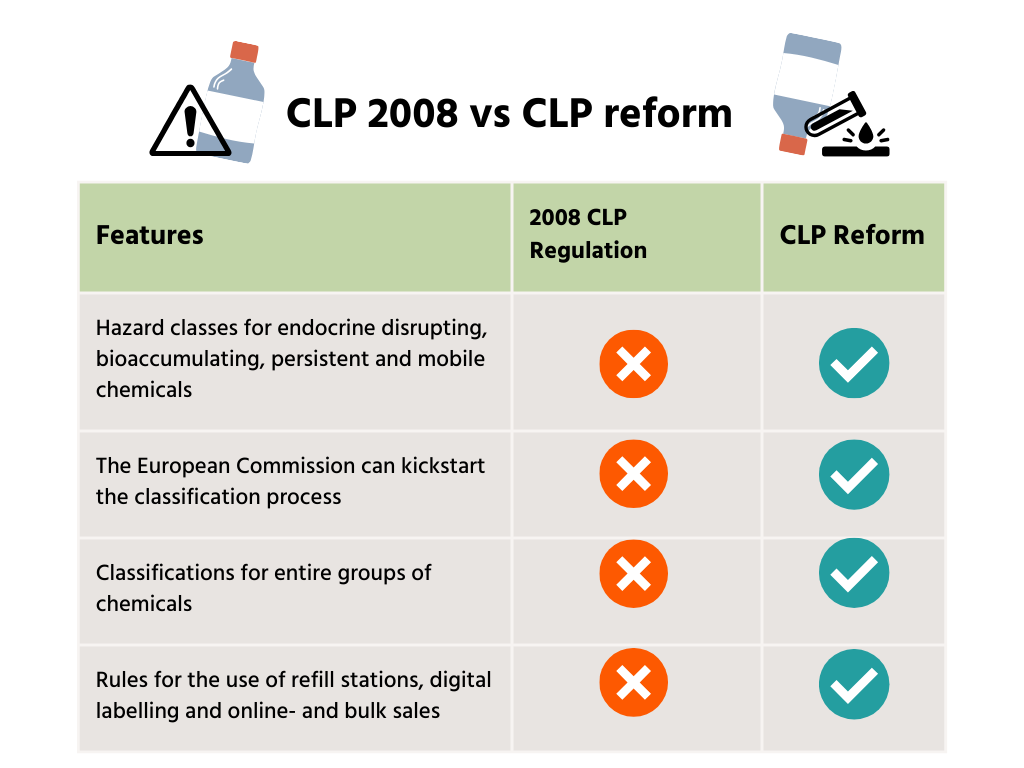
- Introduction of new hazard classes for endocrine disrupting-, bioaccumulating-, persistent- and mobile chemicals and the proper referencing of these new classes throughout the text.
- The 2008 CLP Regulation failed to appropriately address some very important endpoints, such as endocrine disruption, neurotoxicity and immunotoxicity. On the environmental side, hazard classes for persistence, bioaccumulation and toxicity (PBT/vPvB) and persistence, mobility, and toxicity (PMT/vPvM) were also missing. As a consequence, substances with such properties could be placed on the market without being properly identified and without information provision for workers and consumers.
- Allowing classifications for entire groups of chemicals, instead of one-by-one assessments.
- Granting the European Commission the power to initiate hazard classification to speed up the identification of harmful substances.
- Under 2008 legislation, only EU Member States and companies could kickstart the classification process of chemicals. The European Commission was not allowed to propose the classification of a substance, nor propose revisions thereof. Further, the process was slow and not fully transparent.
- Updating the CLP Regulation to suit the demands of the 21st century, with the inclusion of rules for the use of refill stations, digital labelling and online- and bulk sales.
- Clarified rules for packaging of products, including relating to the improved display of hazard icons and of information on the outside of the packaging, to enable informed purchasing choices.
- Incorporation of deadlines and transparency obligations for all stakeholders.
- When it comes to the contentious issues of the addressing of substances with more than one constituent (MOCS), the institutions agreed on an acceptable compromise. Namely, it maintains the Commission’s proposal to align the treatment of MOCS with the approach currently applying to mixtures, with a derogation for plant-based substances plant extracts, for five years, and the commitment of a review of the scientific evidence by the Commission at the end of this period. HEAL strongly supports such a review after 5 years.
In the long term, HEAL supports a system where toxicity studies of a substance or mixture are no longer provided by companies but are commissioned by an independent institution (for example, the European Chemicals Agency or a public authority), and carried out in independent laboratories. In line with the ‘polluter pays’ principle, the costs should be covered by industry and fed into a fund managed by the independent institution in charge.
In HEAL’s view the system would also benefit from improved guidance in relation to transparency and independence in the classification process (for instance through the development of an independence policy for the authorities involved).
Before the new regulation enters into force, the European Parliament and Council have to formally endorse the finalised text, see the current status on the European Parliament website.