HEAL welcomes the call from 250 scientists urging the European Commission to follow scientific recommendations and include provisions to account for the effects of chemical mixtures to better protect health in the upcoming revision of REACH, the EU’s regulation for chemicals.
On 4 October 2023, the European Court of Justice ruled that the 2019 EU-wide ban of the health-harming pesticide chlorpyrifos-methyl remains in force. This blog revisits the long road that led to the decision to ban the substance from the European market in 2019, delves into the related health stakes, and explores the legal challenge that followed the ban.
What is chlorpyrifos-methyl?
Chlorpyrifos-methyl is an organophosphorus pesticide used to control insect pests on a range of crops. Developed by the agrochemical industry in the early 1960s, the first EU-wide approval of chlorpyrifos-methyl dates back to 2006 and stems from its inclusion in European Commission Directive 2005/72/3C (later replaced with Regulation 1107/2009 on the approval of pesticide active substances).
How does this pesticide put people’s health at risk?
Independent scientific studies have shown that chlorpyrifos-methyl may pose serious health risks, including:
- Damage to the proper development and later functioning of the brain (developmental neurotoxicity) from early-life stages onwards. This can translate into neurodevelopmental disorders such as working memory loss, autism, ADHD and decreased IQ.
- DNA damage (genotoxicity): chlorpyrifos-methyl may damage DNA, which can increase the risk of developing cancer.
What is the regulatory history of chlorpyrifos-methyl in Europe?
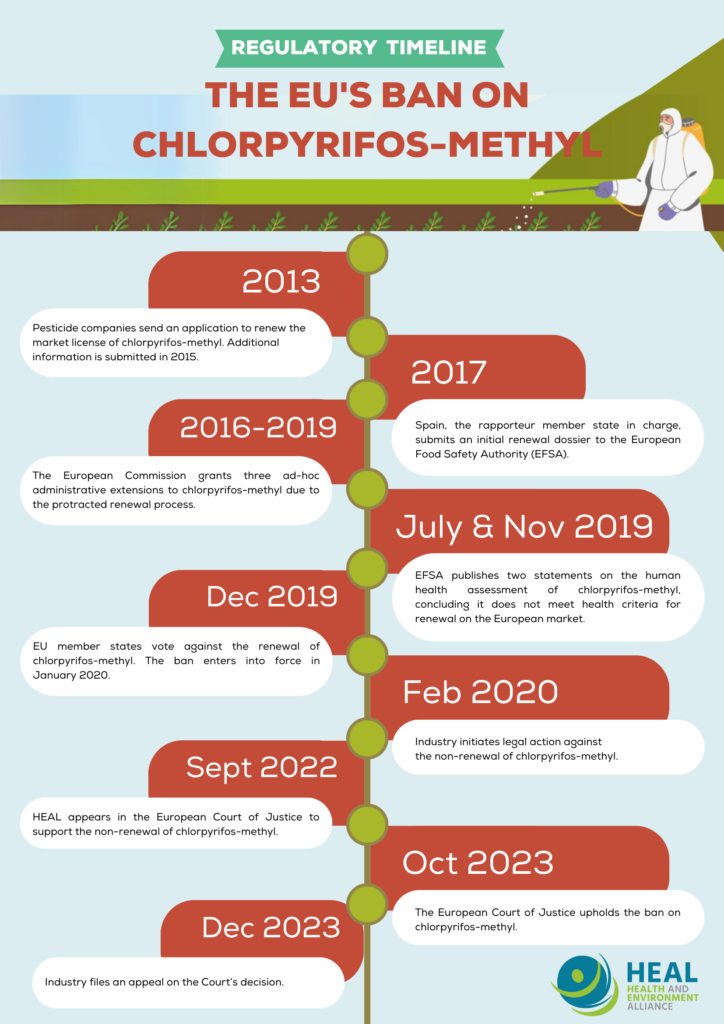
The 2019 ban of chlorpyrifos-methyl was the result of a long regulatory process that spanned over seven years, after the last request for its market renewal. Ahead of the expiry date of the market authorisation of chlorpyrifos-methyl (originally due in 2016), pesticide companies submitted a request to renew its market licence towards European authorities in 2013. As key data was missing from the renewal request, industry was required to submit supplementary information on the pesticide in 2015.
With this information in hand, Spain (the rapporteur member state tasked with overseeing the reauthoritisation process of chlorpyrifos-methyl) submitted an initial renewal dossier to EFSA in 2017.
Due to a lengthy renewal procedure, three ad-hoc one-year administrative extensions were granted by the European Commission after the initial 2016 expiry deadline of chlorpyrifos-methyl’s market licence, leaving people exposed to the harmful chlorpyrifos-methyl for a longer period of time. The reason for these delays can be traced back to data gaps in the dossier regarding several endpoints and flaws revealed during the assessment about the interpretation of important test results regarding developmental neurotoxicity concerns, combined with limited availability of independent scientific literature on the substance at the time. As a result, it was not possible for experts involved in the peer-review of the dossier to rule out that chlorpyrifos-methyl could be associated with developmental neurotoxicity and genotoxicity risks.
In this context, authorities had no other option than to fill data gaps through what is known as a ‘read-across approach’, which consists of using data from a structurally similar substance to predict information about the health impacts of another substance. Experts relied on information available on chlorpyrifos-ethyl, a pesticide closely related to chlorpyrifos-methyl that was also being reassessed at the time.
In August 2019, the European Food Safety Authority (EFSA) issued a statement that concluded that chlorpyrifos-methyl has no possible safety limit and does not meet the human health criteria for renewal on the European market. An updated statement on the available outcomes of the human health assessment of chlorpyrifos-methyl was published by EFSA in November 2019. On that basis, the European Commission issued a proposal for non-renewal of chlorpyrifos-methyl (a proposal for non-renewal of chlorpyrifos-ethyl was issued at the same time based on another EFSA statement confirming the same human health concerns).
In December 2019, representatives from European governments gathering in the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) voted in favour of the Commission’s proposal to ban both chlorpyrifos-methyl and chlorpyrifos-ethyl.
 How did the EU ban of chlorpyrifos-methyl become a legal case, and why did HEAL get involved?
How did the EU ban of chlorpyrifos-methyl become a legal case, and why did HEAL get involved?
Shortly after European governments agreed to ban both substances from the EU market, two agrochemical companies that produce chlorpyrifos-methyl brought legal action against the Commission to challenge the non-renewal of the pesticide. They challenged the quality and legality of the scientific assessment that led to the decision to ban the pesticide.
HEAL requested the right to intervene in this legal challenge and appeared in the European Court of Justice in September 2022 to argue that the decision to ban chlorpyrifos-methyl – as well as chlorpyrifos-ethyl – was backed by scientific evidence and fully in line with applicable EU legal provisions. The French and Danish governments also intervened in the legal procedures to support the ban.
What did the ruling say?
Following almost 12 months of deliberation, the General Court of the EU upheld the 2019 EU-wide ban of the pesticide chlorpyrifos-methyl.
Natacha Cingotti, Health and Chemicals Programme Lead at HEAL commented: “The court ruling upholding the European ban of chlorpyrifos-methyl is a significant victory for the health of Europeans. Neurotoxicity and genotoxicity concerns are not abstract concepts; we are talking about irreversible effects across generations such as the impairment of healthy brain development and functioning – as well as the potential development of cancer.”
HEAL’s press reaction is available here. A more detailed analysis of the court ruling will be provided in the coming weeks.
In December 2023 industry filed an appeal against the ruling.


